Listen to the article – Organized Crime’s Grip on EU Pharmaceutical Industry
Pharmaceutical Frontiers
The pharmaceutical industry in the EU is a crucial component of healthcare, innovation, and economic prosperity. With a significant market share, it serves as one of Europe’s leading high-tech sectors, providing vital medicines and medical devices. European nations, such as Germany, Switzerland, UK, Belgium, and France, invest substantially in research and development (R&D), fostering innovation and medical advancements. Germany, in particular, exhibits a notable surge in R&D activities amounting to USD 9.225 billion, reflecting a commitment to healthcare innovation and industry progress.
EU maintains a substantial presence in the global pharmaceutical market, with a 16.4 percent sales share for new medicines launched between 2017 and 2022.[1] (Fig.1)
Additionally, the prevalence of parallel imports in pharmacy market sales highlights the region’s dynamic pharmaceutical landscape, with countries like Denmark, Netherlands, and Sweden leading in this aspect. The pharmaceutical sector also significantly contributes to employment, with over 860,000 workers, driving economic growth across the region. Market projections indicate continued growth, with the sector expected to reach USD 194.9 billion in the current year, with oncology drugs emerging as the largest market segment. The EU pharmaceutical market is forecasted to experience steady growth, with a projected compound annual growth rate of 6.25 percent from 2024 to 2028, reaching USD 248.4 billion by 2028.[2] Despite global competition, the EU remains a key player, leveraging its innovation and regulatory framework.
How can we help?
Intelligence Solutions
The combination of business, market and strategic intelligence ensures result-driven outcomes for our customers.
Risk management
Risk management through the responsibility of taking risk ownership while ensuring safety and security
Strategic Advisory
The first step in protecting your organization, assets and people is the identification of the risks and threats.
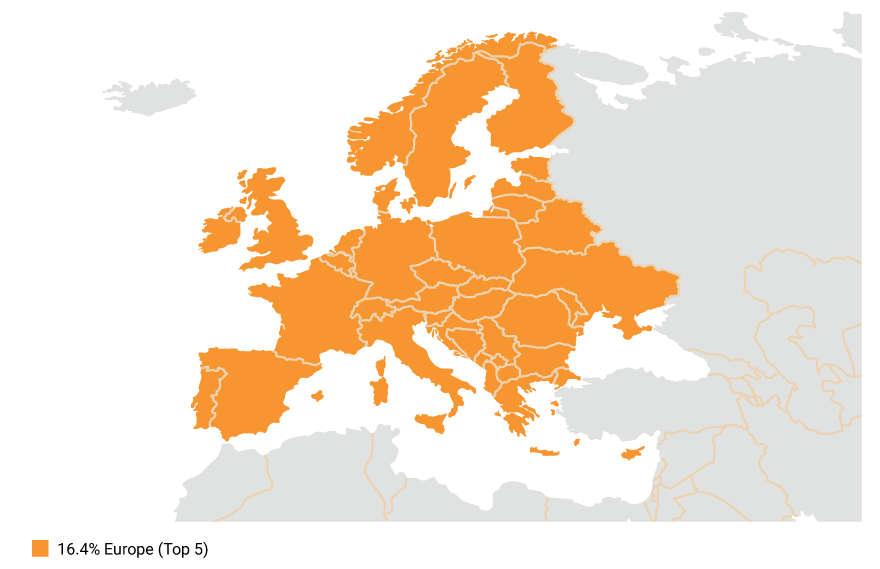
Figure 1. EU maintains a substantial presence in the global pharmaceutical market, with a 16.4 percent sales share for new medicines.
However, the industry faces challenges, notably from organized crime. Organized crime groups engage in illicit activities within the pharmaceutical sector, including counterfeit production, intellectual property (IP) theft, corruption and money laundering. These activities undermine public health, compromise patient safety, and erode trust in legitimate pharmaceutical products, and tarnish industry players’ reputation and operations. The COVID-19 pandemic has exacerbated these risks and provided criminal enterprises an opportunity to abuse the shifted priority focus of law enforcement agencies,[3] exploiting the high demand for medical supplies, including vaccines, by distributing counterfeit or substandard products. This not only endangers public health but also affects economic stability.
To address these challenges, stakeholders must remain vigilant and proactive in combating organized crime within the pharmaceutical industry. By understanding current trends and emerging patterns, stakeholders can develop strategic responses to safeguard their interests and consumer well-being. This necessitates a comprehensive understanding of organized crime’s scope, modus operandi, and impact on the pharmaceutical operations. Through such efforts, stakeholders can mitigate risks and ensure the integrity of the pharmaceutical sector.
01
Unveiling the Underworld
01
Unveiling the Underworld
Various forms of organized crime present significant challenges for the EU pharmaceutical industry. Each aspect interlaps with the others posing unique threats, encompassing illicit trade of counterfeit drugs and controlled substances, IP theft, corruption, and money laundering. These overlapping criminal activities not only endanger patient safety but also undermine the integrity of the pharmaceutical sector and erode public trust.
Counterfeiting
The proliferation of counterfeit pharmaceuticals significantly jeopardizes the integrity and stability of the pharmaceutical industry and compromises public health.
These counterfeit drugs often lack active ingredients or contain harmful substances, endangering patient safety. Organized crime syndicates play a key role in producing, distributing, and trafficking these counterfeit drugs, particularly controlled substances like opioids, exploiting vulnerabilities in the supply chain and circumventing regulatory oversight. This illicit activity fuels addiction and contributes to the opioid crisis in multiple countries, while generating substantial profits for criminal networks. However, the extent of illicit trade in medical products remains uncertain due to undetected and inconsistently reported cases, compounded by a lack of reliable data sources.
Download Report
Organized Crime’s Grip on EU Pharmaceutical Industry
By understanding current trends and emerging patterns, stakeholders can mitigate risks and ensure the integrity of the pharmaceutical sector.
In the EU pharmaceutical industry, counterfeit drug trade attracts criminals due to its lucrative profits, negligible risk of detection, and ease of deceiving consumers. Free trade zones[4] facilitate trafficking, with major source economies including China, India, and Singapore, and transit points including the Persian Gulf, Gulf of Aden, and the Red Sea.[5] Also, Switzerland, US and Iran are commonly identified as points of transit for counterfeited pharmaceuticals destined for the EU.[6] Counterfeiters exploit vulnerabilities in supply chains, infiltrating both illicit channels and the legitimate supply chain, posing a significant hazard to public health, eroding patient confidence in legitimate pharmaceutical companies. Recent seizures, such as those revealed by Europol’s Operation SHIELD IV, underscore the scale of the trade.[7](Fig.2)
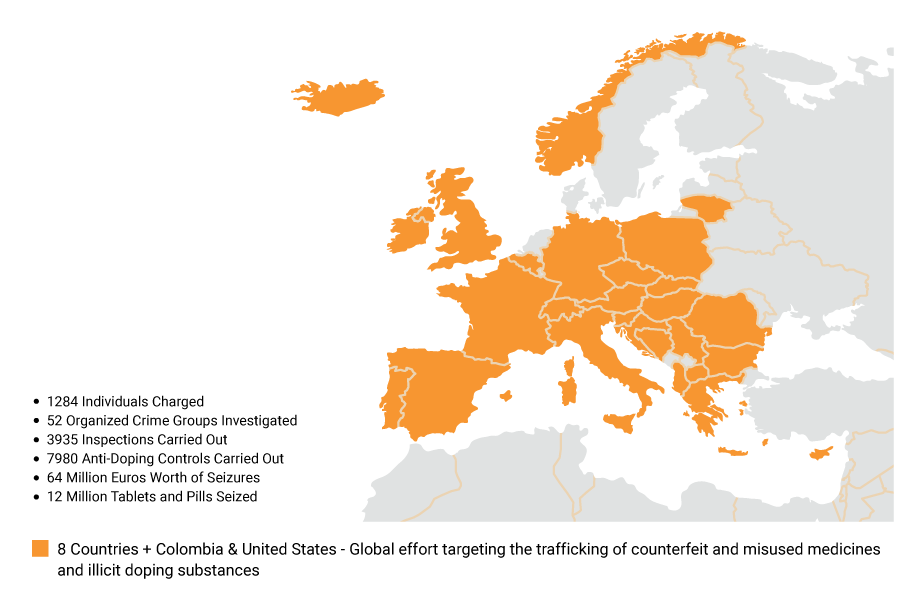
Figure 2. Operation SHIELD IV
Over seven months, the operation led to charges against 1,284 individuals, arrests of 296 suspects, and prosecutions of 988 individuals. Investigations into fifty-two organized crime groups were initiated, resulting in seizures exceeding USD 68.19 million and the dismantling of four underground laboratories. The operation also led to the closure of ninety-two websites and numerous inspections and anti-doping controls, targeting specific illegal operations in Greece and Italy.
Therefore, counterfeiting as a form of organized crime poses a severe threat to the stability and integrity of the pharmaceutical industry, as well as endangering public health. Comprehensive measures are necessary to safeguard patients, protect company credibility, and strengthen regulatory enforcement mechanisms.
Intellectual Property Theft
IP theft presents a major risk to EU’s pharmaceutical industry and is intricately connected to counterfeiting. As a form of organized crime, IP theft undermines innovation, investment, economic stability, and public health.
The pharmaceutical sector, classified among IP-intensive industries, faces heightened vulnerability due to the substantial value and proprietary nature of its products, rendering it an appealing target for organized criminal networks. These networks exploit regulatory disparities and jurisdictional complexities, facilitated by their transnational nature, to unlawfully obtain research and development data, patented formulas, and proprietary technologies. OECD and EU Intellectual Property Office reports reveal that approximately 38 percent of seized counterfeit medicines infringe upon the IP rights of firms registered in the United States, with notable impacts observed in other OECD nations, including Germany, France, and Switzerland.[8] The disruptions caused by the pandemic-induced supply chain disturbances exacerbate these challenges,[9] potentially causing irreversible harm to innovative companies, particularly in light of price dynamics and regulatory frameworks concerning generic substitution drugs.
To combat these illicit activities, law enforcement initiatives such as Operations Ayurveda, Vitra, and Dianu have been deployed within the European pharmaceutical industry. Operation Ayurveda uncovered a counterfeit medicine manufacturing network in Spain sourcing active ingredients from countries like India and China and distributing them under fictitious brands. Operation Vitra focused on disrupting a network importing and selling illegal doping substances, anabolic steroids, and counterfeit medicines in Spain. Operation Dianu, one of Europe’s largest seizures of medicines and prohibited substances, targeted a criminal organization involved in manufacturing, counterfeiting, and distributing medicines and banned substances across Spain, Belgium, and Germany. These operations have resulted in significant arrests and seizures of illicit substances, bolstering efforts to combat theft and organized crime in the pharmaceutical sector. Furthermore, international cooperation, exemplified by initiatives such as the operation against AlphaBay & Hansa, has proven crucial in dismantling criminal marketplaces on the Dark Web and addressing IP theft within the pharmaceutical industry.[10]
In conclusion, the intricate web of IP thefts within the EU’s pharmaceutical industry poses a major threat, intertwining with organized crime networks, which undermines innovation, investment, economic stability, and public health, while exploiting regulatory discrepancies and jurisdictional complexities, thereby necessitating comprehensive strategies to safeguard against such threats.
Corruption
Corruption presents a manifold challenge for EU’s pharmaceutical industry, intertwining legal, economic, and public health concerns. Defined as the misuse of entrusted power for private gain, corruption permeates various facets of society, where the realm of organized crime serves as a fundamental tool for facilitating illicit activities. Within the EU, corruption manifests across the spectrum, ranging from minor instances of bribery to elaborate, multi-million-euro schemes that undermine the rule of law and erode institutional integrity.
At the crux of the issue lies the pervasive influence of corruption, which not only undermines legal frameworks but also exploits vulnerabilities within legitimate business operations. Criminal entities demonstrate remarkable adaptability, capitalizing on crises and utilizing corruption alongside violence and deceit as primary operational tools. The intertwining of corruption with organized crime poses a severe threat to the EU’s efforts to combat illicit activities, with nearly 60 percent of criminal groups reportedly engaging in corrupt practices, as per the SOCTA 2021 report.[11]
Evidence suggests that corruption infiltrates various levels of the pharmaceutical supply chain, from wholesalers and registered pharmacies to healthcare facilities and local markets. Lucrative online networks wield substantial financial influence capable of corrupting individuals within the licit pharmaceutical industry. Criminal elements within the pharmaceutical sector extend to individuals in legitimate positions, such as pharmacists and doctors, who divert medicines or partake in the sale of counterfeit products. These criminal networks exploit weaknesses within the legal supply chain, facilitating the circulation of illicit medications while evading detection.[12]
Cases documented in INTERPOL databases and open sources illustrate instances where pharmacists and medical professionals are incentivized to engage in illicit activities, including filling fraudulent prescriptions or distributing counterfeit medicines for personal gain. Notable cases, such as the involvement of New York pharmacists in the illegal distribution of HIV and AIDS medication, underscore the severity and complexity of the issue. Recent European examples, like the situation in Kosovo,[13] where pharmaceutical products are available without prescriptions, highlight the scale and depth of the problem. Another pertinent example is the corruption probe conducted by the European Public Prosecutor’s Office in Prague, Czech Republic.[14]
This investigation exposed a sophisticated criminal organization allegedly active since 2022, manipulating public procurement processes to favor predetermined suppliers in the provision of medical supplies to hospitals. Key figures within hospitals and contracting authorities are accused of colluding with preferred suppliers, tailoring tendering conditions, and accessing confidential information. Upon securing contracts, suppliers purportedly paid bribes to individuals within the contracting authority, facilitated by an intermediary, thereby compromising the integrity of the procurement process.
The involvement of EU funds, including those from the European Regional Development Fund under the REACT-EU program,[15] underscores the broader implications of corruption on the EU’s economic interests. In fact, the corruption probe led by the European Public Prosecutor in Czech Republic reveal that the illicit activities generated nearly USD one million in damages to EU’s budget.[16] This instance serves as a stark reminder of the urgent need for robust anti-corruption measures and enhanced oversight to preserve the integrity of the EU’s pharmaceutical industry and safeguard public funds and health. Moreover, instances of government and law enforcement officials succumbing to corruption exacerbate the challenge. Cases documented globally, including in regions such as Pakistan and South America, reveal officials colluding with criminal enterprises to embezzle medications or supply expired and counterfeit drugs. Notably, the EU and its pharmaceutical sector are not immune to corruption, as evidenced by the ongoing investigation by the European Public Prosecutor’s Office into allegations involving the EU Commission President and Pfizer’s CEO, including accusations of corruption, conflict of interest, and interference in public functions.[17]
In conclusion, corruption poses a severe challenge to EU’s pharmaceutical industry, linking legal, economic, and public health concerns. Addressing corruption within the EU’s pharmaceutical industry demands a comprehensive approach that navigates the intricate intersections of legal, economic, and public health imperatives.
Money Laundering
Money laundering is another key threat to EU’s pharmaceutical industry, as organized criminal groups, operating on a global scale and generating substantial revenues, have exploited this illicit practice with impunity.
The counterfeit trade serves as a preferred channel for criminal enterprises, intertwining with money laundering alongside other serious crimes such as illicit trade and corruption.[18] During financial crises, money laundering escalates in concern due to various strategies employed by criminals, including the utilization of dormant companies and financially distressed cash-intensive businesses for laundering purposes. Investments in the construction sector and online financial services are also exploited for obscuring the origins of illegal funds.
Recent reports indicate a trend of criminal networks outsourcing money-laundering activities,[19] driven by motives to distance themselves from the predicate offense or to seek expertise on avoiding detection.
How can we help?
Intelligence Solutions
The combination of business, market and strategic intelligence ensures result-driven outcomes for our customers.
Risk management
Risk management through the responsibility of taking risk ownership while ensuring safety and security
Strategic Advisory
The first step in protecting your organization, assets and people is the identification of the risks and threats.
Professional money laundering networks offer a range of services to other criminal networks for a fee, leveraging their expertise and infrastructure to exploit the legal financial industry. These services include currency conversion operations, international transfers through fictitious contracts and invoices, and alternative payment systems, aimed at concealing beneficial ownership. In the EU, money laundering’s magnitude and complexity have often been underestimated. It accounts for approximately two percent of global GDP, equivalent to around USD 1.5 trillion, with significant impacts on the economy. Money laundering facilitates organized crime and terrorism,[20] leading to losses in public revenue and undermining legitimate businesses’ integrity. It distorts market competition, heightens sectoral risks, and threatens financial institutions and entire financial systems. Europol’s findings reveal extensive money laundering activities spanning over eighty countries, with a substantial portion occurring within the EU itself. Nearly half of the most significant criminal networks exclusively launder money within the EU, highlighting the region’s vulnerability. Moreover, sophisticated laundering techniques, from real estate investments to cash-intensive businesses, exacerbate the challenge.
The pharmaceutical industry has become a prime target for money laundering due to its lucrative nature. Criminal entities exploit legal business structures, diverting pharmaceutical products and establishing shell companies to launder illicit funds. Europol’s research shows that 86 percent of the most prominent criminal networks use legal business structures within the pharmaceutical sector for illegal activities. Laundering proceeds not only obscure assets from law enforcement but also generate profits for further criminal enterprises or terrorist activities. The persistent demand for money laundering services stems from the profitability of criminal endeavors and challenges in accessing the formal financial system.
Hence, the convergence of organized criminal activity poses a formidable challenge to the EU’s pharmaceutical sector, necessitating robust measures at multiple levels to combat money laundering and safeguard against associated illicit activities.
02
Counting the Costs
02
Counting the Costs
Organized crime is a key factor negatively influencing pharmaceutical operations, leading to substantial financial losses and jeopardizing public health triggered by counterfeiting, IP theft, corruption and money laundering.
In addition, the intricate network of stakeholders involved in pharmaceutical logistics creates vulnerabilities that criminal groups exploit, necessitating proactive measures to mitigate risks. Despite pharmaceutical companies’ investments in security measures, including technology and compliance with regulations, the agility and adaptability of organized crime networks remain formidable obstacles. The following figure summarizes the level of impact the forms of organized crime activities have on critical aspects of the pharmaceutical industry.(Fig.3)
| R&D | Production | Supply Chain | Reputation | Public Health | Financial Losses | |
| Counterfeiting | Severe | Minor | Major | Severe | Severe | Severe |
| IP Theft | Severe | Minor | Major | Major | Severe | Severe |
| Corruption | Moderate | Major | Severe | Severe | Severe | Major |
| Money Laundering | Insignificant | Moderate | Minor | Major | Moderate | Major |
Figure 3. Organized Crime Risk Assessment on Pharmaceutical industry
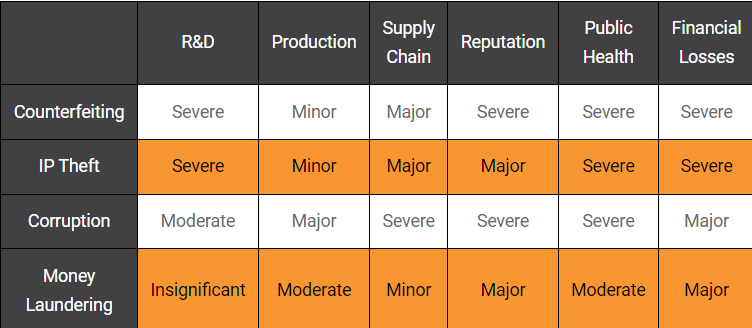 Figure 3. Organized Crime Risk Assessment on Pharmaceutical industry
Figure 3. Organized Crime Risk Assessment on Pharmaceutical industry
Counterfeiting in the pharmaceutical sector has multifaceted impacts, ranging from severe repercussions on R&D investment to public health, reputation and financial losses. Beyond these, major impact extends on the pharmaceutical supply chain, alongside with minor repercussions posing for the manufacturing domain.
Primarily, counterfeiting presents a severe threat to public health as counterfeit drugs often contain harmful ingredients or incorrect dosages, resulting in a staggering toll of fatalities annually, particularly affecting vulnerable demographics. This not only constitutes a grave public health crisis but also inflicts reputational harm upon authentic pharmaceutical companies, particularly in regions like the United States and other OECD nations, where consumer trust is paramount. Against this background, counterfeit products severely tarnish the reputation of pharmaceutical companies.[21] This erosion of trust and brand equity can have severe and long-lasting consequences, as consumers may lose confidence in the authenticity and safety of legitimate pharmaceuticals. Rebuilding trust and restoring brand reputation requires substantial effort and resources.

Moreover, the economic toll of counterfeit pharmaceuticals reverberates across multiple sectors. The EU faces an approximate 4.4 percent loss in sales, amounting to USD 10.87 billion, due to counterfeiting.[22] Governments sustain substantial revenue losses, estimated at USD 1.81 billion annually within the EU, due to the proliferation of counterfeit pharmaceuticals. Concurrently, healthcare systems bear the burden of treating patients harmed by these fraudulent medications, incurring considerable financial expenses.
For the pharmaceutical industry though, the financial ramifications of counterfeiting are substantial, encompassing revenue loss, litigation costs, and damage to brand value. The sale of counterfeit pharmaceuticals undermines legitimate market revenues, resulting in severe financial losses for pharmaceutical companies. Additionally, legal proceedings to combat counterfeiting and defend IP rights incur substantial expenses. Likewise, the erosion of brand value diminishes the market position and competitiveness of pharmaceutical companies, further exacerbating financial losses.
In addition, counterfeiting severely affects R&D efforts by potentially deterring investment in the development of genuine pharmaceuticals. The presence of counterfeit products undermines the incentive for innovation and diminishes revenue prospects for legitimate pharmaceutical companies. Consequently, the industry may witness a reduction in the pursuit of groundbreaking treatments and advancements. While the direct impact on production processes may be relatively minor, counterfeiting disrupts downstream activities in the supply chain. Finished product manufacturing may experience disruptions and quality control challenges as counterfeit drugs infiltrate legitimate production channels, albeit to a lesser extent compared to other areas.
Also, counterfeiting exerts a major influence on the pharmaceutical supply chain, primarily through the infiltration of counterfeit drugs into legitimate distribution channels. This infiltration leads to regulatory non-compliance, necessitates product recalls, and compromises patient safety. The presence of counterfeit products within the supply chain disrupts the integrity and reliability of pharmaceutical distribution networks.[23] Besides, the EU pharmaceutical and related sectors suffer job losses, affecting over 80,000 positions, thereby exacerbating unemployment rates and economic instability within the region. The impact of counterfeit pharmaceuticals extends beyond the EU, with the OECD estimating damages at USD 461 billion, equivalent to 2.5 percent of the world trade.[24] While developed economies may experience counterfeit rates as low as 0.2 percent of the market, less developed nations face a disproportionately higher incidence, with counterfeit rates reaching as high as thirty percent, as evidenced by a UNODC report on anti-malarial drugs in Africa.[25]
In summary, counterfeiting poses severe and wide-ranging effects on the EU pharmaceutical industry, impacting R&D, production, supply chain integrity, reputation, public health, and financial viability. Addressing this complex issue requires concerted efforts from stakeholders across the industry, regulatory bodies, and law enforcement agencies to mitigate risks and safeguard the integrity of pharmaceutical products and the well-being of consumers.
Intellectual property theft, particularly in the form of counterfeit medicines, is encompassing both direct and indirect economic repercussions. This form of organized crime undermines the integrity of the pharmaceutical sector, severely impacting various facets of the industry, including R&D and issues about public health. The financial impacts are also severe, encompassing loss of revenue, competitive disadvantage, and legal expenses associated with defending IP rights. Intellectual property crimes in the EU constitute 5.8 percent of EU imports with USD 127.5 billion worth of counterfeit goods entering the EU market.[26]
Pharmaceutical companies incur significant financial losses when competitors unlawfully exploit their proprietary information to gain market share or produce counterfeit products. These sales shortfalls directly diminish the revenue streams of legitimate pharmaceutical companies, hindering their ability to invest in R&D.
Legal proceedings to enforce IP rights further drain resources and disrupt business operations, exacerbating financial strain on companies. Sales losses are substantial across EU Member States, with Italy and Spain particularly affected by significant absolute impacts. Germany and France also experience adverse effects, albeit to a lesser extent.[27] Besides, IP theft has a severe impact on R&D efforts, as it erodes the competitive advantage of pharmaceutical companies.
Download Report
Organized Crime’s Grip on EU Pharmaceutical Industry
By understanding current trends and emerging patterns, stakeholders can mitigate risks and ensure the integrity of the pharmaceutical sector.
The theft of proprietary information and research findings discourages future investment in innovation, as companies face heightened risks of their discoveries being exploited by competitors without appropriate compensation. This discouragement hampers the development of novel treatments and therapies, ultimately impeding progress in addressing unmet medical needs. While the direct impact on physical production facilities may be minor, IP theft primarily affects product formulation and manufacturing processes. Competitors may illicitly obtain proprietary formulas, manufacturing techniques, or process specifications, enabling them to replicate pharmaceutical products bypassing the investment in R&D.
Moreover, IP theft has a major impact on the pharmaceutical supply chain, particularly through its association with counterfeit drugs. Stolen IP may be used to produce counterfeit pharmaceuticals, which can infiltrate legitimate supply chains. The presence of counterfeit drugs compromises product integrity, regulatory compliance, and patient safety, posing significant risks to public health and eroding trust in the pharmaceutical industry. This disruption reverberates throughout the economy as well, affecting various sectors that supply goods and services to the pharmaceutical industry. The resultant economic downturn further compounds the challenges faced by legitimate businesses, exacerbating the overall impact of IP theft on the EU economy. The job losses incurred in the legitimate pharmaceutical industry due to decreased sales serve as a stark reminder of the economic toll inflicted by IP theft and counterfeit medicines. Germany bears the brunt of these losses, followed by Italy, France, and Spain. The erosion of employment opportunities not only undermines individual livelihoods but also dampens consumer confidence and contributes to broader socio-economic instability.
Furthermore, IP theft severely damages the reputation of pharmaceutical companies as innovators and reliable providers of safe and effective medicines. The perception of a company’s integrity and commitment to innovation is tarnished when its intellectual property is unlawfully appropriated by competitors or counterfeiters. This damage to reputation can have long-term consequences, impacting consumer trust, investor confidence, and stakeholder relationships. Also, IP theft poses severe risks to public health. Counterfeit pharmaceuticals produced using stolen intellectual property likely contain substandard or harmful ingredients, incorrect dosages, or lack efficacy, endangering the health and well-being of patients. The prevalence of counterfeit drugs undermines public confidence in the safety and efficacy of pharmaceutical products, exacerbating public health concerns.
In conclusion, IP theft poses substantial threats to the EU pharmaceutical industry, affecting R&D, production, supply chain integrity, reputation, public health, and financial viability. Addressing this pervasive issue requires robust measures to safeguard IP rights, strengthen regulatory enforcement, and enhance collaboration among stakeholders to protect the integrity of pharmaceutical products and preserve public health.
Corruption imposes significant economic costs affecting R&D, production, supply chain integrity, reputation, public health, and financial viability to the EU pharmaceutical industry. Corruption is a key feature of organized crime.[28] Despite distorting competition by favoring companies with political connections or willingness to engage in unethical practices over those adhering to regulatory standards, corruption further undermines fair market competition and stifles innovation, as resources are diverted towards securing favorable treatment rather than investing in R&D.

Inefficiencies in resource allocation and diminished incentives for innovation impede the industry’s long-term growth and competitiveness, translating into lost economic opportunities for the EU pharmaceutical sector. While corruption’s direct impact on R&D investment may be less pronounced, it can still exert a moderate influence by influencing research priorities or compromising the integrity of clinical trials. Corruption diverts resources towards less critical areas or skew research findings, potentially hindering scientific progress and innovation in the pharmaceutical sector. Corruption within manufacturing processes has a major impact on the production of pharmaceuticals. It can lead to the production of substandard or falsified products, endangering patient safety and regulatory compliance. Bribery or unethical practices may compromise quality control measures, resulting in the manufacture of pharmaceuticals that fail to meet safety and efficacy standards, thereby jeopardizing public health. Corruption throughout the pharmaceutical supply chain has severe repercussions. It undermines regulatory oversight mechanisms, facilitating the distribution of counterfeit drugs and low-quality products. Bribery or collusion may enable the infiltration of counterfeit products into legitimate distribution channels, posing severe risks to patient safety and eroding trust in the integrity of pharmaceutical supply chains.
Furthermore, corruption engenders inefficiencies in healthcare delivery by diverting resources away from patient care towards illicit payments or kickbacks. It may result in the distribution of substandard or counterfeit drugs that lack proper quality control and regulatory oversight. Patients consuming such products are exposed to potentially harmful substances, incorrect dosages, or ineffective treatments, jeopardizing their health and well-being. Corruption exacerbates existing public health challenges and undermines efforts to ensure access to safe and effective medicines.
Association with corrupt practices severely damages the reputation of pharmaceutical companies, undermines public trust in healthcare systems, discouraging patients from seeking treatment and undermining the credibility of medical professionals and institutions. The proliferation of illicit online pharmacies for instance, is poised to exacerbate the level of corruption within the pharmaceutical industry. Fictitious prescriptions and fraudulent medical documentation are anticipated to become increasingly prevalent, posing significant challenges to regulatory authorities and law enforcement agencies tasked with safeguarding public health and maintaining the integrity of the pharmaceutical sector. Evidently, the erosion of trust can have profound economic consequences, as it undermines patient adherence to treatment regimens, impedes disease prevention efforts, and exacerbates public health crises. Consequently, pharmaceutical companies may suffer financial losses due to product withdrawals, damage to brand reputation, loss of market share and legal penalties. Pharmaceutical companies implicated in corrupt practices may face hefty fines or legal sanctions, resulting in substantial financial losses. Moreover, corruption damages brand reputation and erodes consumer confidence, leading to loss of market share and revenue. Addressing corruption requires robust anti-corruption measures and enforcement actions to mitigate financial risks and safeguard the integrity of the pharmaceutical industry.
In summary, corruption poses severe threats to the EU pharmaceutical industry, affecting R&D, production, supply chain integrity, reputation, public health, and financial viability. Combating corruption demands concerted efforts to strengthen regulatory oversight, promote transparency and accountability, and foster a culture of integrity within the pharmaceutical sector.
Money laundering presents nuanced implications to the pharmaceutical industry within EU, as trade-based money laundering activities are expected to escalate in the coming period. Within this context, free trade zones emerge as critical focal points for the analysis, given their key role in attracting foreign investment and driving economic growth, particularly in developing countries. However, the allure of lightly regulated zones within the EU can inadvertently facilitate illicit financial activities, including money laundering, counterfeiting, and smuggling, thereby jeopardizing the integrity of the pharmaceutical sector. As a hub for pharmaceutical production, distribution, and investment, EU provides an attractive target for criminal enterprises seeking to launder illicit funds. The sophisticated infrastructure and extensive network of pharmaceutical companies and suppliers within the EU offer ample opportunities for money launderers to exploit regulatory loopholes and obscure the origins of illicit proceeds. On the other hand, enhanced EU legislation in the field of anti-money laundering and the resulting increase of financial supervision in the banking sector has made it more difficult for criminal networks to introduce illicit proceeds into the legal economy through traditional banking channels. As a result, money laundering attempts are likely to be displaced towards sectors with nascent controls or limited oversight. This could include the use of underground remittance agencies, alternative banking platforms, international trade, and anonymous virtual currencies. The use of cryptocurrencies is also an area of growing concern, due to the absence of a common regulatory regime and the level of anonymity these products offer.
Seen through the lens of the pharmaceutical industry operations, this form of organized crime has an insignificant direct impact on R&D activities within. However, indirectly, it may influence the allocation of resources by diverting funds away from legitimate investments in innovation and research, though such effects are typically minimal. In context of production, money laundering can exert a moderate impact. Investments in illicit manufacturing facilities or the acquisition of raw materials for counterfeit drug production may occur through laundered funds. While not directly affecting the production processes of legitimate pharmaceutical companies, these activities contribute to the proliferation of counterfeit drugs, undermining patient safety, regulatory compliance and brand reputation. As far as the legitimate pharmaceutical supply chain is concerned, money laundering activities generally have a minor impact. However, the interconnected nature of the global pharmaceutical supply chain exacerbates the risk of money laundering and EU is not immune to it as well.
How can we help?
Intelligence Solutions
The combination of business, market and strategic intelligence ensures result-driven outcomes for our customers.
Risk management
Risk management through the responsibility of taking risk ownership while ensuring safety and security
Strategic Advisory
The first step in protecting your organization, assets and people is the identification of the risks and threats.
Free trade zones, which serve as conduits for international trade and investment, can inadvertently facilitate the movement of illicit funds across borders. The lack of robust regulatory oversight and coordination between government authorities and private operators within these zones creates vulnerabilities that can be exploited by criminal syndicates seeking to launder money through legitimate business transactions.
Association with money laundering significantly undermines the reputation of pharmaceutical companies. Involvement in such illicit financial activities raises concerns about ethical conduct, regulatory compliance, and integrity. This damage to reputation can erode trust among stakeholders, including consumers, healthcare professionals, and regulatory authorities, impacting market perception and competitiveness. Money laundering’s impact on public health is moderate, primarily through its indirect contribution to the proliferation of counterfeit drugs. As previously noted, laundered funds may support the production and distribution of counterfeit pharmaceuticals lacking proper quality control and regulatory oversight, posing risks to public health and patient safety. While not directly causing harm, money laundering indirectly facilitates activities that endanger public health.
Lastly, money laundering activities result in major financial impacts on pharmaceutical companies that extend beyond the immediate financial losses incurred. Moreover, the diversion of illicit funds into the legitimate economy can distort market competition, stifle innovation, and ultimately compromise EU’s ability to address pressing public health challenges. Conversely, regulatory penalties, legal expenses, and damage to brand reputation stemming from involvement in money laundering can lead to significant financial losses, and expectedly, association with illicit financial activities deters investors and partners, further exacerbating financial challenges for affected companies.
In conclusion, while money laundering may not directly impact certain aspects of the EU pharmaceutical industry, its effects are felt indirectly through contributions to counterfeit drug proliferation, reputational damage, public health risks, and financial losses. Mitigating these risks requires robust anti-money laundering measures, enhanced regulatory oversight, and industry-wide collaboration to uphold integrity and compliance standards within the sector.
03
Investment Vigilance
03
Investment Vigilance
For investors in the EU pharmaceutical industry, the implications of organized crime underscore the importance of thorough due diligence, risk assessment, and proactive mitigation strategies.
Risk Assessment and Due Diligence
Investors must conduct comprehensive risk assessments to evaluate the exposure of pharmaceutical companies to counterfeiting, IP theft, corruption, and money laundering, as the most influential forms of organized crime. This involves scrutinizing companies’ supply chain management practices, regulatory compliance efforts, and reputational risks. Thorough due diligence processes should be employed when evaluating potential investments, considering factors such as the company’s geographic footprint, distribution channels, and partnerships with third-party vendors.[29]
Financial Impact
Organized crime activities can have significant financial implications for investors in the EU pharmaceutical industry. Counterfeiting, IP theft, corruption, and money laundering most likely result in revenue loss, regulatory fines, legal expenses, and damage to brand value. Therefore, investors should carefully assess the financial stability and resilience of pharmaceutical companies to withstand potential losses resulting from organized crime. Additionally, investors may need to factor in the costs of implementing robust anti-counterfeiting measures, enhancing cybersecurity defenses, and strengthening compliance frameworks to mitigate these risks effectively.
Reputational Risks
Association with organized crime activities tarnishes the reputation of pharmaceutical companies, leading to erosion of consumer trust, stakeholder confidence, and brand equity. Investors should consider the reputational risks posed by investing in companies with perceived vulnerabilities to organized crime. Maintaining a positive reputation is essential for long-term shareholder value and market competitiveness. Therefore, investors ought to prioritize investments in pharmaceutical companies with strong ethical cultures, transparent governance structures, and demonstrated commitment to compliance and integrity.
Regulatory Compliance
Regulatory scrutiny of EU pharmaceutical industry is heightened due to the potential public health and safety implications of the above-mentioned organized crime activities. Investors should assess the regulatory compliance record of accomplishment of pharmaceutical companies, including adherence to good manufacturing practices, pharmacovigilance requirements, and anti-bribery and anti-corruption regulations. Non-compliance with regulatory standards can further result in legal and financial penalties, product recalls, and reputational damage, impacting investor returns and shareholder value.
Long-Term Sustainability
Investors should consider the long-term sustainability of investments in the EU pharmaceutical industry in light of organized criminal activities. Companies that proactively address these risks through robust risk management practices, investments in technology and innovation, and collaboration with law enforcement agencies and regulatory authorities are better positioned to protect shareholder value and maintain market competitiveness over the long term. Therefore, investors should prioritize companies with a demonstrated commitment to corporate social responsibility, ethical business conduct, and proactive engagement with stakeholders.
In summary, investors in the EU pharmaceutical industry need to carefully assess the implications of organized crime risks on financial performance, reputation, regulatory compliance, and long-term sustainability. By incorporating these considerations into investment decision-making processes, investors would be better positioned to mitigate risks and identify opportunities for value creation in this critical sector.

ARTICLE | 30 PAGES